IOF Blog
Intermediate Hip & Knee Orthobiologic Injections Course FAQ
During this one-day course, attendees will have the opportunity to advance their knowledge of orthobiologics and injection techniques for the hip and knee under ultrasound and fluoroscopy. This course will be led by IOF expert instructors Ariana DeMers, DO, RMSK and Mary Ambach, MD.
IOF Member of the Week: Dr. Henry Stiene
IOF Member of the Week: Dr. Chris Williams
IOF Regulatory Warning: Exosome Products
Case Study: Cervical Spine Fusion Treated with PRP
IOF Launches Ethics Committee; How to Report Unlawful Sale of Medical Products
FDA Response Regarding Chara Biologics Products
IOF Expands Course Offerings to Florida and Washington
Statement from IOF President on the New York Times Article “An Unproven Cure”
IOF 2019 Conference Videos Available for Purchase
IOF 2019 Conference Videos Available for Purchase The Interventional Orthopedics Foundation’s 2019 annual conference, #IOF2019: Controversies in Orthobiologics, brought together national and global experts to discuss the latest original research and controversial topics in the field of regenerative and interventional orthopedic medicine. Over the course of two days, presenters shared the latest original research […]
IOF President Receives $590,000 Award to Treat Meniscal Injuries in Military Personnel
Interventional Orthopedics Foundation President Dr. Gerard Malanga, MD has received a research grant to treat active-duty military personnel. Along with Dr. Trevor Dyson-Hudson, MD and Dr. Nathan Hogaboom, PhD, Dr. Malanga received a $590,000 award from the Geneva Foundation for the use of Autologous Micro-Fragmented Adipose Tissue to Treat Meniscal Injuries in Active-Duty Military Personnel. […]
Dr. Ariana DeMers Joins the IOF Board
We are excited to announce the addition of Ariana DeMers, D.O. to the Interventional Orthopedics Foundation Board. “We are thrilled to add Dr. DeMers to our board,” said IOF Chairman Gerard Malanga, M.D. “Her extensive interventional orthopedics experience coupled with her drive to innovate and educate physicians and patients about this rapidly growing speciality makes […]
FDA Warning
The U.S. Food & Drug Administration (FDA) has issued a warning letter to Cord for Life, an Altamonte Springs, Florida company that has sold umbilical-cord-blood products to stem cell companies. The FDA also issued 20 letters to companies instructing them to stop selling stem cell products that are not FDA approved. All IOF members should […]
Birth Tissue Products Consensus and IOF Webinar Recording
The aggressive marketing of birth tissues has been one of the most controversial issues in orthobiologics in recent years. On February 18, 2019, a consensus statement provided by physicians, academics and regulatory experts was published concerning the aggressive marketing of these non-viable birth tissues as live “stem cell” products to cure chronic disease. As we […]
Q&A with IOF Instructor Dr. James Leiber
We recently sat down with Dr. James Leiber, DO, one of IOF’s esteemed instructors and the founder and medical director of New Regeneration Orthopedics of Florida, to find out about how he became involved in IOF and regenerative medicine. IOF: How did you become involved with the Interventional Orthopedics Foundation? JL: I’ve been an affiliate […]
Your Right to Use Autologous Orthobiologics May be Threatened
We Need to Organize in Denver in February 2017 [dk-video id=”0zb53w_PyaM” button=”white” show-title=”true” width=”” align=””] Many of you may know that the FDA held a hearing this fall on stem cell clinics and new guidelines that could restrict your ability to use orthobiologics such as autologous bone marrow concentrate and fat grafts. That was followed […]
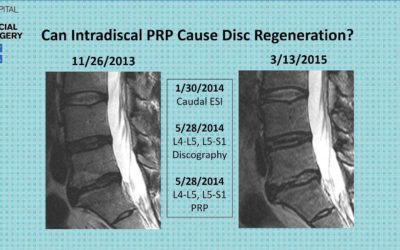
Can Platelet Rich Plasma (PRP) Regenerate a Degenerative Disc?
This slide was provided by Dr. Greg Lutz who will discuss his two year findings from his PRP Lumbar Disc Study at #IOF2017 in Denver, Colorado. What do you think? Do you also see lumbar discs regenerate with PRP or BMC? Attend the Interventional Orthopedics Foundation’s 2017 Annual Conference to learn more about this thought-provoking […]
Learn About Platelet Rich Plasma (PRP) Dosage in Tendons at #IOF2017
If you’re using 3-5X PRP in tendons you may be under dosing certain patients.
Two articles have been published suggesting that higher concentrations of PRP cause tenocyte inhibition. Given that both studies had serious design flaws, Interventional Orthopedics Foundation (IOF) scientists repeated these experiments.
Interventional Orthopedics Foundation Announces Fellowship Grants
As part of its commitment to furthering the quality of education in the field of interventional orthopedics, the Interventional Orthopedics Foundation is proud to announce that it has awarded two prestigious universities and medical centers in the United States for the 2016-2017 academic year. The grants were awarded through the Foundation and provided a total […]
Regenerative Orthopedics Offer New Hope to Injured Soldiers
Future of Personal Health reports on how regenerative orthopedic procedures are helping soldiers recover from traumatic injuries.
Summary of Patient Registry Database Statistics and Benefits
The Interventional Orthopedics Foundation (IOF) Patient Registry Database is a tool developed to assist physician providers, with long-term patient tracking for practice improvement, outcomes management, adverse effects, and efficient, successful participation in benchmarking. This document provides an At-a-Glance look at the benefits for doctors and patients alike, as well as specifics on the information collected. […]
Wounded Warrior Program Update
Another Wounded Warrior was treated with his own stem cells at the facility of one of our participating members. This patient is a former Coast Guard who suffered hip and knee injuries while performing his duties as a helicopter-based search and rescue operator. With 24 practices across the US, the IOF is opening doors for […]
Platelet Rich Plasma (PRP) and Why the IOF is Studying its Effects on Cultured Cells
By Dustin Berger, M.S. Platelets are small cell fragments within our blood that play important roles in the wound healing process. In addition to being responsible for stopping blood flow at a site of injury, platelets are loaded full of beneficial growth factors. Increased interest in platelet biology began over 40 years ago, with cell […]
Interventional Orthopedics Foundation Receives Additional Funding to Further Advance the Organization
BROOMFIELD, Colo.–(BUSINESS WIRE)–The Interventional Orthopedics Foundation (IOF), a 501c3 non-profit organization has announced a $960,000 gift from cable television executive John C. Malone, to continue its dedication to educate and serve as a resource to physicians and the public who seek to understand the growing field of interventional orthopedics, which includes the development and availability […]
Companies Respond to IOF Findings Related to Placental Tissue-Derived Products
A reference for information regarding placental derived products. This data includes company follow-up and statements / responses to our December 16, 2015 press release regarding an absence of stem cells in placental tissue-derived products.
Interventional Orthopedics Foundation Shines Light on False Claims about Stem Cell Products
BROOMFIELD, Colo.–(BUSINESS WIRE)–The Interventional Orthopedics Foundation (IOF), a 501c3 non-profit dedicated to providing education about regenerative orthopedic medicine, recently announced the results of independent lab tests showing an absence of live stem cells contained within placental tissue-derived products. Based on the findings of their research, many physicians are defrauding consumers by claiming that they are […]
In Vitro Evaluation of Injectable, Placental Tissue-Derived Products for Orthopedic Applications
2015 Fellowship Grants Awarded!
September 1, 2015 – As part of its commitment to furthering the quality of education in the field of interventional orthopedics, the Interventional Orthopedics Foundation is proud to announce that it has awarded two prestigious universities and medical centers in the United States for the 2015-2016 academic year. The grants were awarded through the Foundation and […]
Contact Us
IOF Business Office:
4950 S. Yosemite St. F2 #313
Greenwood, CO 80111
Phone: 303-469-4431
Fax: 303-479-2608